![SOLVED: BALANCE THESE REDOXEQUATIONS: Mn(NO3)z NaBiO3 HNO3 HMnO4 Bi(NO3)a NaNO3 Hzo NaCrOz NaCIO NaOH NazCrO4 NaCl Hzo KMnO HzC204 HzSO4 MnSO4 KzSOa CO2 Hzo HTML Editorn] X E E B 4 A " SOLVED: BALANCE THESE REDOXEQUATIONS: Mn(NO3)z NaBiO3 HNO3 HMnO4 Bi(NO3)a NaNO3 Hzo NaCrOz NaCIO NaOH NazCrO4 NaCl Hzo KMnO HzC204 HzSO4 MnSO4 KzSOa CO2 Hzo HTML Editorn] X E E B 4 A "](https://cdn.numerade.com/ask_images/82b4778276d44dbd9252d5cb26c3ae2d.jpg)
SOLVED: BALANCE THESE REDOXEQUATIONS: Mn(NO3)z NaBiO3 HNO3 HMnO4 Bi(NO3)a NaNO3 Hzo NaCrOz NaCIO NaOH NazCrO4 NaCl Hzo KMnO HzC204 HzSO4 MnSO4 KzSOa CO2 Hzo HTML Editorn] X E E B 4 A "

Bi (NO3)3·5H2O and cellulose mediated Cu-NPs — A highly efficient and novel catalytic system for aerobic oxidation of alcohols to carbonyls and synthesis of DFF from HMF - ScienceDirect

Plausible mechanism for Bi(NO3)2.5H2O catalyzed synthesis of 8-alkyl... | Download Scientific Diagram

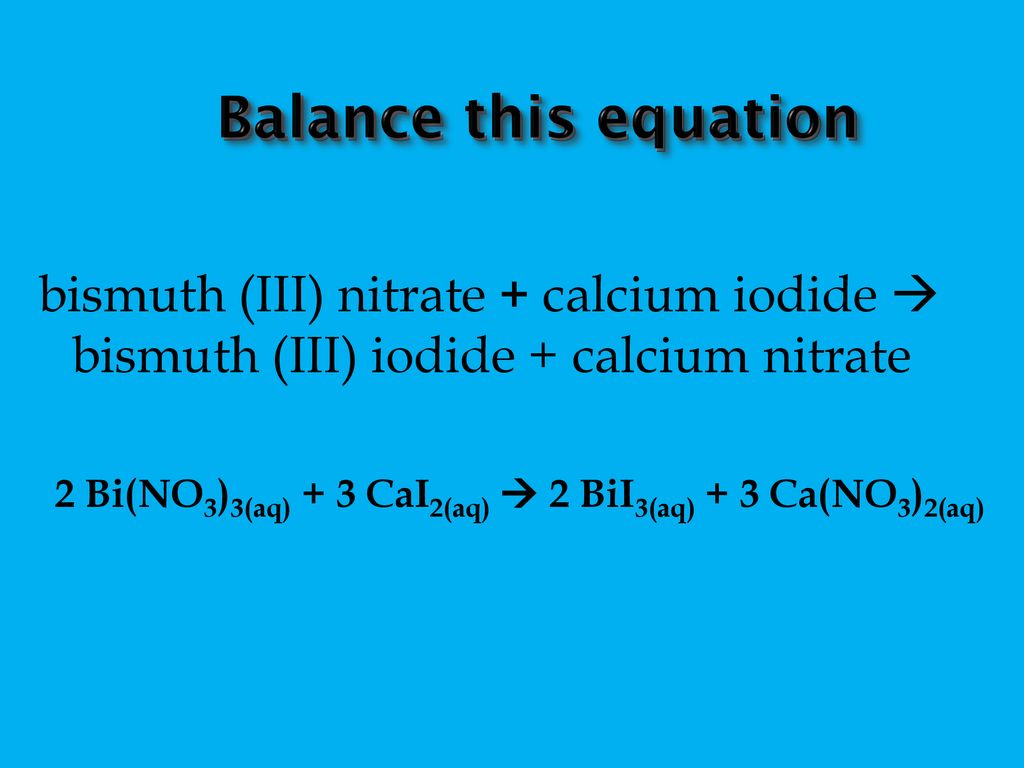
The gradual addition of KI solution to Bi (NO3)3 solution initially produces a dark brown precipitate which dissolves in excess of KI to give yellow solution. write the chemical equations for the

Gradual addition of KI solution to Bi(NO3)(3) solution initially produces a dark brown precipitate which dissolves in excess of KI to give a clear yellow solution. Write chemical equation for the above

Bi(NO3)3·5H2O: An efficient catalyst for one-pot synthesis of 3-((aryl)(diethylamino)methyl)-4-hydroxy-2H-chromen-2-ones and biscoumarin derivatives - ScienceDirect

Bismuth Nitrate Crystal Bi (NO3) 3 Crystal with CAS No 10361-44-1 - China Bismuth Nitrate and Bismuth Nitrate Crystal

Hydrolysis Studies on Bismuth Nitrate: Synthesis and Crystallization of Four Novel Polynuclear Basic Bismuth Nitrates | Inorganic Chemistry

Simple soluble Bi(iii) salts as efficient catalysts for the oxidation of alkanes with H2O2 - Catalysis Science & Technology (RSC Publishing)
Gradual addition of KI solution of Bi(NO3)3 solution initially produces a dark brown precipitate - Sarthaks eConnect | Largest Online Education Community
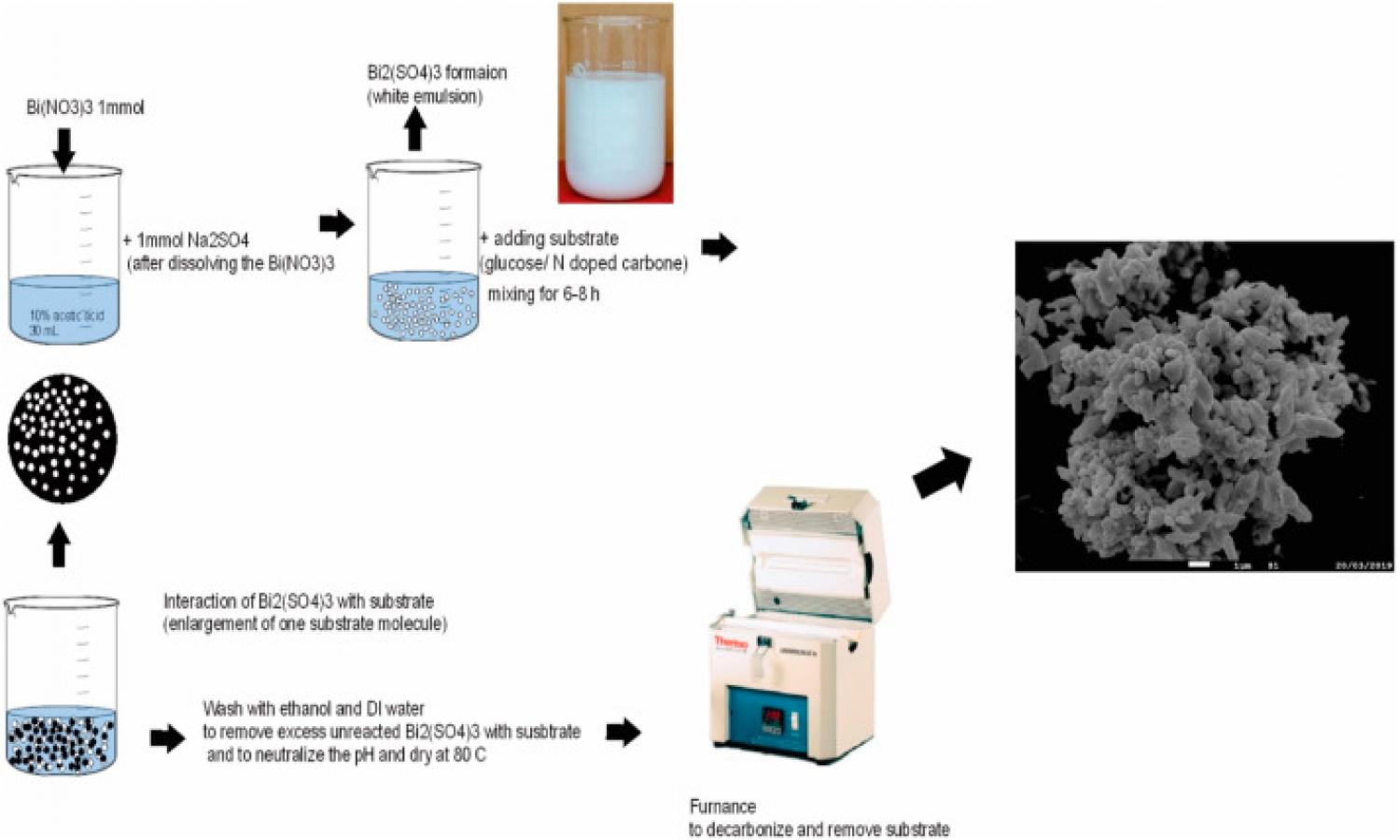

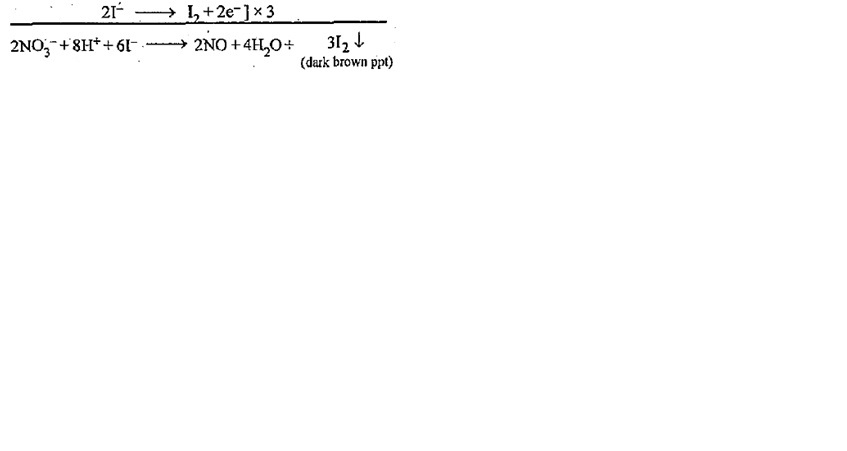
.jpg)
![PDF] Hydrothermal synthesis of sodium and potassium bismuth titanates | Semantic Scholar PDF] Hydrothermal synthesis of sodium and potassium bismuth titanates | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7ab5f4365aefc6be9fd46d2c68477b872ddd589b/3-Table1-1.png)

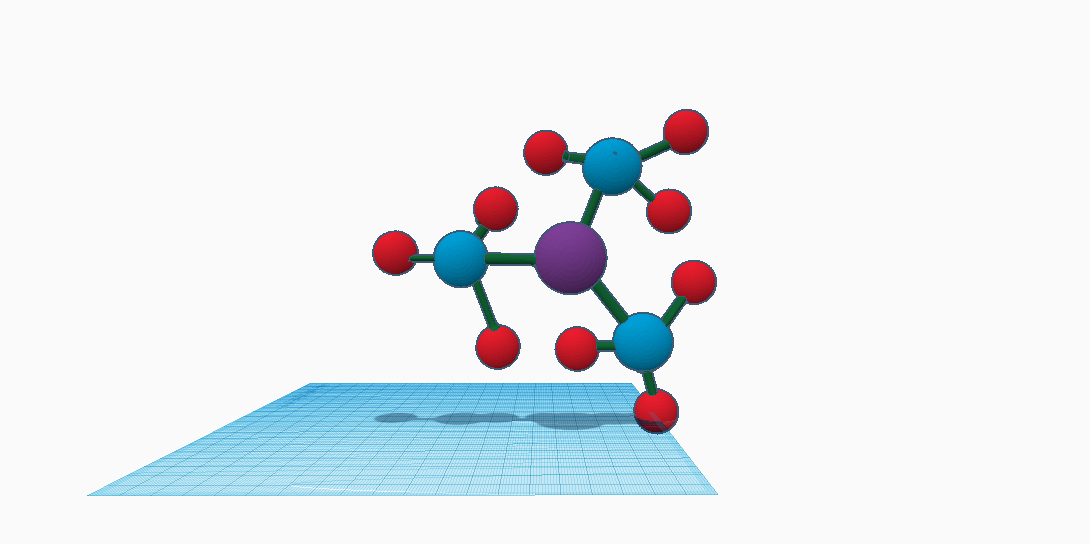
![Structure of [Bi(NO 3 ) 3 (H 2 O) 3 ]*18-crown-6. | Download Scientific Diagram Structure of [Bi(NO 3 ) 3 (H 2 O) 3 ]*18-crown-6. | Download Scientific Diagram](https://www.researchgate.net/publication/329597257/figure/fig2/AS:703233244790785@1544675279163/Structure-of-BiNO-3-3-H-2-O-3-18-crown-6.png)
